Role of confinement, molecular connectivity and flexibility in entropic driven surface segregation of polymer–colloid mixtures
Abstract
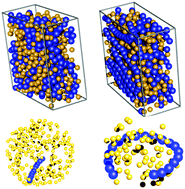
Relative surface affinity between polymers and colloids is leveraged in many applications like filtration, adhesion, bio-sensing, etc. The surface affinity is governed by both enthalpic (relative interactions between the species and surface) and entropic (excluded volume) effects. Neglecting enthalpic effects, i.e. for purely athermal systems, entropy is the only driving force that controls the surface affinity of the species in a binary or multi-component mixture. Many intensive (relative size of colloids, chain length, equilibrium bond angle, chain flexibility) and extensive (confinement, temperature) factors can dramatically change the entropy of the system and thus enhance or decrease the surface affinities of the constituent species. In this article, we use coarse grained metropolis Monte Carlo simulations to delineate the role of these factors in entropic surface segregation in a binary mixture of polymers and colloids. At low number densities, excluded volume effects are negligible and we do not observe any entropic driven surface segregation. Therefore our system of interest is binary polymer–colloid mixtures at moderate to high number densities where excluded volume effects are predominant. Our results indicate that for flexible polymer chains, the surface is always enriched with colloids compared to polymers and this effect is enhanced for longer polymer chains. The configurational entropy of the flexible polymers is significantly reduced near the surface and therefore they prefer to stay in the bulk (away from the surface). However this behavior can be completely reversed by introducing a large degree of confinement and making the chains relatively rigid (less flexible). Our results show that polymer segregation of long stiff chains in slit pore geometry is driven by nematization near the surface while looping of polymers is observed under a large degree of confinement. We observe that for longer polymer chains with an equilibrium bond angle (θ = π), both confinement and chain stiffness enhance the surface segregation of polymers relative to colloids. However, the segregation behavior within a confinement is dependent on the polymer chain length. The surface segregation of polymers is dramatically decreased for chains with equilibrium bond angles 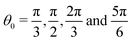 independent of chain length and flexibility due to excluded volume effects and inefficient packing near the surface.
independent of chain length and flexibility due to excluded volume effects and inefficient packing near the surface.


 Please wait while we load your content...
Please wait while we load your content...