Diffusion-limited dissolution of calcium carbonate in a hydrogel
Abstract
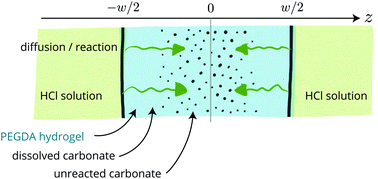
In the process of fabricating porous polymers, we use dispersed calcite as a sacrificial solid that generates porosity after dissolution. To do this, we trap calcite particles in a hydrogel, dissolve the particles and dry the hydrogel; here, we describe in detail the dissolution kinetics. We prepare PEGDA [poly(ethylene glycol)-diacrylate] water solutions loaded with micron-sized calcite particles (containing mostly calcium carbonate) up to about 30% volume fraction; these dispersions are photo-polymerized into hydrogels as flat and shallow monoliths with a typical thickness of ≈100 μm and a lateral extent on the order of 1 cm. These soft hydrogels are then soaked into an acidic solution (HCl) which induces the dissolution of the carbonates. The dissolution fronts remain sharp throughout the dissolution and progress inward in a diffusive manner. Such a kinetics is well described numerically using a mean-field diffusion–reaction model where the diffusion of the acid strongly limits the process.


 Please wait while we load your content...
Please wait while we load your content...