Enhanced stability of crosslinked and charged unimolecular micelles from multigeometry triblock copolymers with short hydrophilic segments: dissipative particle dynamics simulation†
Abstract
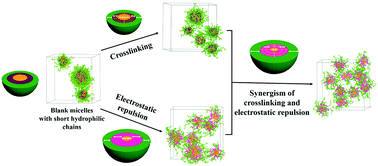
High micellar stability and well-performed drug loading and release are two conflicting factors for unimolecular micelles as an ideal drug delivery system. Achieving the formation of unimolecular micelles with short hydrophilic blocks is a challenging and promising approach to solve this bottleneck and limitation of current unimolecular micelle systems. In this work, dissipative particle dynamics (DPD) simulation is used to study the synergetic effect of crosslinking and electrostatic repulsion on stability of unimolecular micelles and to analyze the micro-mechanism and factors influencing this synergetic stabilization strategy. The strategy can generate unimolecular micelles with extremely high stability for various supramolecular polymers with short hydrophilic chains. Protonation of DEAEMA blocks leads to a large improvement in micellar hydrophilicity. The protonated middle layer further shrinks through crosslinking to produce the largest charge density, enlarging the electrostatic repulsion between colloidal particles. Additionally, the crosslinking and protonation treatment maximizes the extension degree of hydrophilic EO segments due to the increasing steric hindrance and poor compatibility between DEAHEMA and EO blocks. In this study, the relation between shrinkage degree of hydrophobic cores and stability of unimolecular micelles is first reported. The above-mentioned transition of micellar structures and properties results in the maximum degree of core shrinkage (Rg of MMA blocks) corresponding to the high stability of unimolecular micelles. Further study shows that the increasing cyclization degree, the mode of end cyclization, and the crosslinking and electrostatic repulsion of the middle layer all exert favorable effects on the stability of unimolecular micelles due to controlled shrinkage of hydrophobic cores.


 Please wait while we load your content...
Please wait while we load your content...