Propofol adsorption at the air/water interface: a combined vibrational sum frequency spectroscopy, nuclear magnetic resonance and neutron reflectometry study†
Abstract
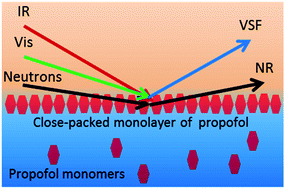
Propofol is an amphiphilic small molecule that strongly influences the function of cell membranes, yet data regarding interfacial properties of propofol remain scarce. Here we consider propofol adsorption at the air/water interface as elucidated by means of vibrational sum frequency spectroscopy (VSFS), neutron reflectometry (NR), and surface tensiometry. VSFS data show that propofol adsorbed at the air/water interface interacts with water strongly in terms of hydrogen bonding and weakly in the proximity of the hydrocarbon parts of the molecule. In the concentration range studied there is almost no change in the orientation adopted at the interface. Data from NR show that propofol forms a dense monolayer with a thickness of 8.4 Å and a limiting area per molecule of 40 Å2, close to the value extracted from surface tensiometry. The possibility that islands or multilayers of propofol form at the air/water interface is therefore excluded as long as the solubility limit is not exceeded. Additionally, measurements of the 1H NMR chemical shifts demonstrate that propofol does not form dimers or multimers in bulk water up to the solubility limit.


 Please wait while we load your content...
Please wait while we load your content...