Li-decorated carbyne for hydrogen storage: charge induced polarization and van't Hoff hydrogen desorption temperature†
Abstract
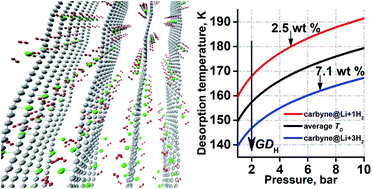
We have studied carbyne as a promising hydrogen storage material. Density functional theory simulations with vdW corrections have been used to investigate lithium sorption on carbyne and the interaction of pristine and Li-functionalized carbon chains with molecular hydrogen. We showed that Li adatoms at small concentrations stay atomically dispersed on carbyne, donating 0.9e to the chain. Moreover, in the presence of Li, hydrogen adsorption energy increases by more than 5 times in comparison with pristine carbyne. Overall, up to three hydrogen molecules per Li adatom have an adsorption energy close to the range of 200–600 meV per H2, which is necessary for effective sorption/desorption cycles. The resulting theoretical uptake (7.1 wt%) is higher than the U.S. Department of Energy's ultimate goal (6.5 wt%). The calculated van't Hoff desorption temperatures exceed considerably the boiling point of liquid nitrogen. Our results confirm the potential of Li-decorated carbyne for hydrogen storage.


 Please wait while we load your content...
Please wait while we load your content...