Electrochemical hydrogen production on a metal-free polymer†
Abstract
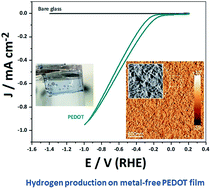
The exploration for true electrocatalytic reactions at organic conducting polymer electrodes, including chemisorption of a reactant and desorption of a product, is receiving renewed interest due to the profound implications it could have on low-cost large area electrochemical energy technology. Here, we finalize the debate about the ability of an organic electrode, more specifically poly(3,4-ethylenedioxythiophene) (PEDOT), to be an electrocatalyst for hydrogen production. This paper proves and covers fundamental studies of the hydrogen evolution reaction (HER) on PEDOT films. Both theory based on DFT (Density Functional Theory) and experimental studies using electrochemical techniques and operando mass spectrometry suggest a Volmer–Heyrovsky mechanism for the actual HER on PEDOT. It is shown that PEDOT reaches an exchange current density comparable to that of metals (i.e. Cu, Ni, and Au) and in addition does not form passivating oxide layers or suffer from chemical corrosion in acidic media. Finally, an electrolyzer stack using the organic polymer electrode demonstrates HER performance in real applications.
- This article is part of the themed collection: 2019 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...