Vapor-fed electrolysis of water using earth-abundant catalysts in Nafion or in bipolar Nafion/poly(benzimidazolium) membranes†
Abstract
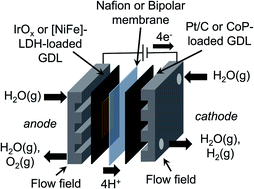
Vapor-fed electrolysis of water has been performed using membrane-electrode assemblies (MEAs) incorporating earth-abundant catalysts and bipolar membranes (BPMs). Catalyst films containing CoP nanoparticles, carbon black, and Nafion were synthesized, characterized, and integrated into cathodes of MEAs. The CoP-containing MEAs exhibited stable (>16 h) vapor-fed electrolysis of water at room temperature at a current density of 10 mA cm−2 with 350 mV of additional overvoltage relative to MEA's formed from Pt/C cathodic electrocatalysts due to slower hydrogen-evolution reaction kinetics under vapor-fed conditions and fewer available triple-phase boundaries in the catalyst film. Additionally, catalyst films containing a [NiFe]-layered double hydroxide ([NiFe]-LDH) as well as a hydroxide ion conductor, hexamethyl-p-terphenyl poly(benzimidazolium) (HMT-PMBI), were synthesized, characterized, and integrated into the anodes of the MEAs. The [NiFe]-LDH-containing MEAs exhibited overvoltages at 10 mA cm−2 that were similar to those of IrOx-containing MEAs for vapor-fed electrolysis of water at room temperature. A BPM was formed by pairing Nafion with HMT-PMBI, resulting in a locally alkaline environment of HMT-PMBI to stabilize the [NiFe]-LDH and a locally acidic environment to stabilize the CoP. BPM-based MEAs were stable (>16 h) for vapor-fed electrolysis of water at room temperature at a current density of 10 mA cm−2, with a change in the pH gradient of 1 unit over 16 h of electrolysis for IrOx-containing MEAs. The stability of [NiFe]-LDH-based MEAs under vapor-fed conditions was dependent on the catalyst film morphology and resulting BPM interface, with stable operation at 10 mA cm−2 achieved for 16 h. All MEAs exhibited a drift in the operating voltage over time associated with dehydration. These results demonstrate that earth-abundant catalysts and BPMs can be incorporated into stable, room-temperature, vapor-fed water-splitting cells operated at 10 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...