Shifting the equilibrium of methanol synthesis from CO2 by in situ absorption using ionic liquid media†
Abstract
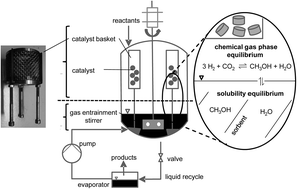
Renewable energy sources such as wind and solar power generate fluctuating electric energy. To bridge energy-lean times and to utilize the temporal surplus of electricity production large amounts of energy must be stored. One promising approach is the chemical storage in methanol that is formed from renewable H2 from water electrolysis and CO2. In our contribution we demonstrate a way to increase the methanol yields in CO2 hydrogenation significantly by in situ sorption of methanol and water in alkali salt-doped ionic liquids (ILs). The reaction and product sorption take place in a single reactor vessel which shifts the methanol yield from the thermodynamic equilibrium of 25.3% at 75 bar (H2 : CO2 = 3 : 1) and 250 °C up to 60%.


 Please wait while we load your content...
Please wait while we load your content...