Binary nickel iron phosphide composites with oxidized surface groups as efficient electrocatalysts for the oxygen evolution reaction†
Abstract
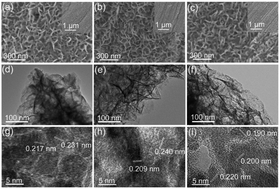
Electrocatalytic water splitting has become an effective approach to produce hydrogen as a clean energy source. Developing electrocatalysts for the oxygen evolution reaction (OER) with high efficiency and low cost is crucial for hydrogen energy systems due to the sluggish kinetics of OER. Herein, we have synthesized binary NiFeOH, NiFeO and NiFeP two-dimensional (2D) nanosheets on carbon cloth (CC) via a facile hydrothermal method combined with phosphorization and oxidation treatments. The porous binary NiFeP nanosheets exhibit an OER overpotential of 281 mV to achieve 20 mA cm−2 and a small Tafel slope of 74 mV dec−1, which are lower than those of NiFeO (336 mV and 81 mV dec−1). After the continuous OER operation for 50 h, the NiFeP-32/CC exhibits a small degradation of the current density (88% remains). The results indicate that large amounts of NiO and NiOOH phases formed on the NiFeP nanosheets during the OER process. The electrocatalytic activity for oxidized NiFeP with NiO and NiOOH phases is higher than that of pure NiFeO and NiFeOH, indicating that the synergistic effects among the NiFeP, NiO and NiOOH phases could improve the OER activity.


 Please wait while we load your content...
Please wait while we load your content...