Tailoring the composition of a one-step electrodeposited Co,Ni/Co,Ni(OH)2 composite coating for a highly active hydrogen evolution electrode†
Abstract
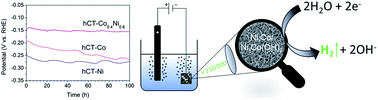
Low hydrogen evolution overpotential, along with high stability during operation and ease of preparation from inexpensive raw materials, is a crucial characteristic of an effective electrode for large-scale hydrogen generation by alkaline water electrolysis. Herein, we present a method of preparation of a low-cost electrode, applying as a substrate a carbon fiber textile derived from a technical carbon fiber reinforced polymer. Noble-metal free composite films composed of cobalt–nickel alloy and amorphous Co,Ni(OH)2 phases are obtained by a one-step electrodeposition process. Deposition is optimized in terms of the applied potential as well as metal ion concentration and Co/Ni ratio in an electroplating bath. The experimental results indicate that the hCT-Co0.4Ni0.6 electrode possesses the lowest HER overpotential of 150 mV at a current density of 10 mA cm−2 in 1.0 M KOH, with negligible activity loss within 100 h of galvanostatic operation. A significant decrease of the overpotential and electrode stability boost are achieved thanks to the precise control of the bimetallic electrode composition, which affects the morphology, electrocatalytically active surface area and electronic properties of the material.


 Please wait while we load your content...
Please wait while we load your content...