Revisiting the use of electrolyte additives in Li–S batteries: the role of porosity of sulfur host materials
Abstract
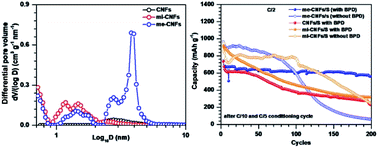
In this work, we demonstrate the important role of porosity of the sulfur host material in the efficient functioning of the biphenyl-4,4′-dithiol (BPD) electrolyte additive in Li–S batteries. We compare the electrochemical performance of Li–S cells fabricated using non-porous (CNFs), micro-porous (mi-CNFs), and micro-mesoporous carbon nanofibers (me-CNFs) as sulfur hosts. me-CNFs/S cathodes exhibit a stable specific capacity with 83% capacity retention at C/2 after 200 cycles and 90% retention at C/5 after 150 cycles, whereas mi-CNFs/S and CNFs/S cathodes retain close to only 30% capacity after 200 cycles. We investigate the role of porosity using two approaches – Li+ diffusion coefficient and shuttle-current measurements. The me-CNFs/S cathodes show a relatively higher Li+ ion diffusion coefficient during reduction and oxidation processes thus indicating a low concentration of BPD–Sx2− species in the electrolyte. Furthermore, the me-CNFs/S cathodes indicate a relatively lower shuttle effect compared to the other two cathodes, further validating the presence of a lower concentration of polysulfides in the electrolyte. Based on the size of the BPD–Sx2− complex, we infer that the use of mesoporous CNFs is essential for achieving long-term cycling. Our results show that the integration of suitable porous host materials with functional electrolyte additives presents a promising approach for developing high-performance Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...