Aqueous microdroplets containing only ketones or aldehydes undergo Dakin and Baeyer–Villiger reactions†
Abstract
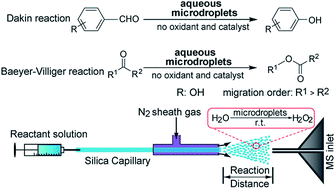
The Dakin and Baeyer–Villiger (BV) oxidation reactions require addition of peroxides as oxidants and an acid or a base as a catalyst. Reaction times range from hours to days to obtain target products. Previously, we reported that hydrogen peroxide (H2O2) is spontaneously generated in water microdroplets without any added chemicals or applied electrical potential. Here, we report that the Dakin and BV reactions occur in modest yields within milliseconds in aqueous microdroplets at room-temperature without the addition of external peroxides and catalysts. H2O2 generation is the result of the special environment of the microdroplet surface, which promotes water autoionization. We find that increasing the content of water and decreasing the droplet size improve the product yield of the Dakin and BV reactions, supporting the contention that the amount of H2O2 generated in aqueous microdroplets could induce the two reactions and the reactions occur at or near the air–water interface of the microdroplet surface.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...