Chiral cis-iron(ii) complexes with metal- and ligand-centered chirality for highly regio- and enantioselective alkylation of N-heteroaromatics†
Abstract
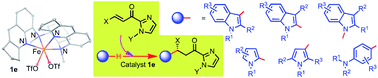
Iron-catalyzed highly regio- and enantioselective organic transformations with generality and broad substrate scope have profound applications in modern synthetic chemistry; an example is herein described based on cis-FeII complexes having metal- and ligand-centered chirality. The cis-β FeII(N4) complex [FeII(L)(OTf)2] (L = N,N′-bis(2,3-dihydro-1H-cyclopenta-[b]quinoline-5-yl)-N,N′-dimethylcyclohexane-1,2-diamine) is an effective chiral catalyst for highly regio- and enantioselective alkylation of N-heteroaromatics with α,β-unsaturated 2-acyl imidazoles, including asymmetric N1, C2, C3 alkylations of a broad range of indoles (34 examples) and alkylation of pyrroles and anilines (14 examples), all with high product yields (up to 98%), high enantioselectivity (up to >99% ee) and high regioselectivity. DFT calculations revealed that the “chiral-at-metal” cis-β configuration of the iron complex and a secondary π–π interaction are responsible for the high enantioselectivity.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...