Secondary amine selective Petasis (SASP) bioconjugation†
Abstract
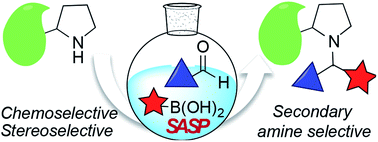
Selective modification of proteins enables synthesis of antibody-drug conjugates, cellular drug delivery and construction of new materials. Many groups have developed methods for selective N-terminal modification without affecting the side chain of lysine by judicious pH control. This is due to lower basicity of the N-terminus relative to lysine side chains. But none of the methods are capable of selective modification of secondary amines or N-terminal proline, which has similar basicity as lysine. Here, we report a secondary amine selective Petasis (SASP) reaction for selective bioconjugation at N-terminal proline. We exploited the ability of secondary amines to form highly electrophilic iminium ions with aldehydes, which rapidly reacted with nucleophilic organoboronates, resulting in robust labeling of N-terminal proline under biocompatible conditions. This is the first time the Petasis reaction has been utilized for selective modification of secondary amines on completely unprotected peptides and proteins under physiological conditions. Peptide screening results showed that the reaction is highly selective for N-terminal proline. There are no other chemical methods reported in literature that are selective for N-terminal proline in both peptides and proteins. This is a multicomponent reaction leading to the synthesis of doubly functionalized bioconjugates in one step that can be difficult to achieve using other methods. The key advantage of the SASP reaction includes its high chemoselective and stereoselective (>99% de) nature, and it affords dual labeled proteins in one pot. The broad utility of this bioconjugation is highlighted for a variety of peptides and proteins, including aldolase and creatine kinase.
- This article is part of the themed collection: Most popular 2019-2020 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...