Controlled scrambling reactions to polyphosphanes via bond metathesis reactions†
Abstract
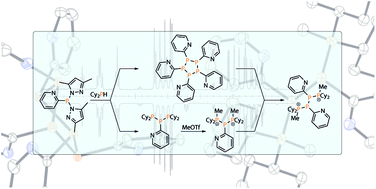
Triphosphanes R′2PP(R)PR′2 (9a,c: R = Py; 9b R = BTz), 1,3-diphenyl-2-pyridyl-triphospholane 9d and pentaphospholanes (RP)5 (13: R = Py; 18: R = BTz) are obtained in high yield of up to 98% from the reaction of dipyrazolylphosphanes RPpyr2 (5: R = Py; 6: R = BTz; pyr = 1,3-dimethylpyrazolyl) and the respective secondary phosphane (R′2PH, R′ = Cy (9a,b), tBu (9c); PhPH(CH2)2PHPh (9d)). The formation of derivatives 9a–d proceeds via a condensation reaction while the formation of 13 and 18 can only be explained by a selective scrambling reaction. We realized that the reaction outcome is strongly solvent dependent as outlined by the controlled scrambling reaction pathway towards pentaphospholane 13. In our further investigations to apply these compounds as ligands we first confined ourselves to the coordination chemistry of triphosphane 9a with respect to coinage metal salts and discussed the observation of different syn- and anti-isomeric metal complexes based on NMR and X-ray analyses as well as quantum chemical calculations. Methylation reactions of 9a with MeOTf yield triphosphan-1-ium Cy2MePP(Py)PCy2+ (10+) and triphosphane-1,3-diium Cy2MePP(Py)PMeCy22+ (112+) cations as triflate salts. Salt 11[OTf]2 reacts with pentaphospholane 13 in an unprecedented chain growth reaction to give the tetraphosphane-1,4-diium triflate salt Cy2MePP(Py)P(Py)PMeCy22+ (19[OTf]2) via a P–P/P–P bond metathesis reaction. The latter salt is unstable in solution and rearranges via a rare [1,2]-migration of the Cy2MeP-group followed by the elimination of the triphosph-2-en-1-ium cation [Cy2MePPPMeCy2]+ (20+) to yield a novel 1,4,2-diazaphospholium salt (21[OTf]).


 Please wait while we load your content...
Please wait while we load your content...