Covalent bonds in positron dihalides†
Abstract
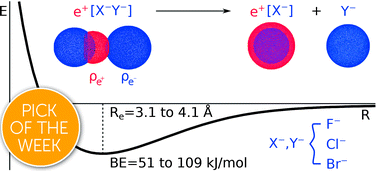
We report a computational study on homo- and heteronuclear e+[X−Y−] compounds formed by two halide anions (X−, Y− = F−, Cl−, Br−) and one positron. Our results indicate the formation of energetically stable positronic molecules in all cases. Analysis of the electron and positron densities points out that the formation of positron covalent bonds underlies the stabilization of the otherwise repelling dihalides, revealing that positronic bonding can reach far beyond the previously addressed e+[H−H−] molecule [J. Charry, M. T. do N. Varella and A. Reyes, Angew. Chem. Int. Ed., 2018, 57, 8859–8864.]. To a significant extent, the properties of the positron dihalides are similar to those of the purely electronic analogs, e−[A+B+], molecular cations with isoelectronic atomic cores (A+, B+ = Na+, K+, Rb+) bound by one electron. The positron bonds in the e+[X−Y−] complexes are however stronger than those in the isoelectronic e−[A+B+] counterparts, as the former have shorter bond lengths and higher bond energies. While an energy decomposition analysis points out that both electronic and positronic bonds essentially arise from electrostatic interactions, the more stable positron bonds are partly due to the higher polarizabilities of the dihalide anions, and partly to more significant contributions from correlation and relaxation effects.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry, 2019 Chemical Science HOT Article Collection and 2019 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...