Influence of the heavy-atom effect on singlet fission: a study of platinum-bridged pentacene dimers†
Abstract
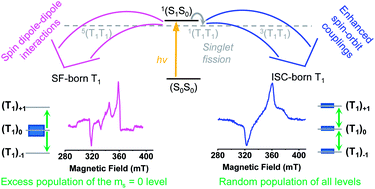
The process of singlet fission (SF) produces two triplet excited states (T1 + T1) from one singlet excited exciton (S1) and a molecule in its ground state (S0). It, thus, possesses the potential to boost the solar cell efficiency above the thermodynamic Shockley–Queisser limit of 33%. A key intermediate in the SF mechanism is the singlet correlated triplet pair state 1(T1T1). This state is of great relevance, as its formation is spin-allowed and, therefore, very fast and efficient. Three fundamentally different pathways to formation of 1(T1T1) have been documented so far. The factors that influence which mechanism is associated with which chromophore, however, remain largely unknown. In order to harvest both triplet excitons independently, a decorrelation of the correlated triplet pair state to two individual triplets is required. This second step of the SF process implies a change in the total spin quantum number. In the case of a dimer, this is usually only possible if the coupling between the two pentacenes is sufficiently weak. In this study, we present two platinum-bridged pentacene dimers in which the pentacenes are coupled strongly, so that spin-decorrelation yielding (T1 + T1) was initially expected to be outcompeted by triplet–triplet annihilation (TTA) to the ground state. Both platinum-bridged pentacene dimers undergo quantitative formation of the (T1T1) state on a picosecond timescale that is unaffected by the internal heavy-atom effect of the platinum. Instead of TTA of (T1T1) to the ground state, the internal heavy-atom effect allows for 1(T1T1)–3(T1T1) and 1(T1T1)–5(T1T1) mixing and, thus, triggers subsequent TTA to the (T1S0) state and minor formation of (T1 + T1). A combination of transient absorption and transient IR spectroscopy is applied to investigate the mechanism of the (T1T1) formation in both dimers. Using a combination of experiment and quantum chemical calculations, we are able to observe a transition from the CT-mediated to the direct SF mechanism and identify relevant factors that influence the mechanism that dominates SF in pentacene. Moreover, a combination of time-resolved optical and electron paramagnetic resonance spectroscopic data allows us to develop a kinetic model that describes the effect of enhanced spin–orbit couplings on the correlated triplet pair state.


 Please wait while we load your content...
Please wait while we load your content...