Isothiourea-catalysed enantioselective Michael addition of N-heterocyclic pronucleophiles to α,β-unsaturated aryl esters†
Abstract
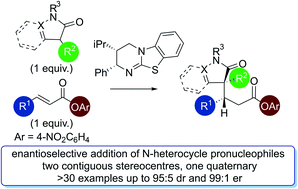
The isothiourea-catalysed enantioselective Michael addition of 3-aryloxindole and 4-substituted-dihydropyrazol-3-one pronucleophiles to α,β-unsaturated p-nitrophenyl esters is reported. This process generates products containing two contiguous stereocentres, one quaternary, in good yields and excellent enantioselectivities (>30 examples, up to > 95 : 5 dr and 99 : 1 er). This protocol harnesses the multifunctional ability of p-nitrophenoxide to promote effective catalysis. In contrast to previous methodologies using tertiary amine Lewis bases, in which the pronucleophile was used as the solvent, this work allows bespoke pronucleophiles to be used in stoichiometric quantities.
- This article is part of the themed collection: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis


 Please wait while we load your content...
Please wait while we load your content...