Anion carriers as potential treatments for cystic fibrosis: transport in cystic fibrosis cells, and additivity to channel-targeting drugs†
Abstract
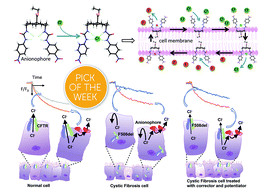
Defective anion transport is a hallmark of the genetic disease cystic fibrosis (CF). One approach to restore anion transport to CF cells utilises alternative pathways for transmembrane anion transport, including artificial anion carriers (anionophores). Here, we screened 22 anionophores for biological activity using fluorescence emission from the halide-sensitive yellow fluorescent protein. Three compounds possessed anion transport activity similar to or greater than that of a bis-(p-nitrophenyl)ureidodecalin previously shown to have promising biological activity. Anion transport by these anionophores was concentration-dependent and persistent. All four anionophores mediated anion transport in CF cells, and their activity was additive to rescue of the predominant disease-causing variant F508del-CFTR using the clinically-licensed drugs lumacaftor and ivacaftor. Toxicity was variable but minimal at the lower end. The results provide further evidence that anionophores, by themselves or together with other treatments that restore anion transport, offer a potential therapeutic strategy for CF.
- This article is part of the themed collections: Most popular 2019-2020 supramolecular chemistry articles, 2019 Chemical Science HOT Article Collection and 2019 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...