Ni-catalyzed 1,2-benzylboration of 1,2-disubstituted unactivated alkenes†
Abstract
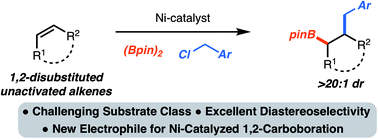
Nickel-catalyzed 1,2-carboboration of alkenes is emerging as a useful method for chemical synthesis. Prior studies have been limited to only the incorporation of aryl groups. In this manuscript, a method for the 1,2-benzylboration of unactivated alkenes is presented. The reaction combines readily available alkenes, diboron reagents and benzylchlorides to generate synthetically versatile products with control of stereochemistry. The utility of the products as well as the mechanistic details of the process are also presented.
- This article is part of the themed collection: Most popular 2018-2019 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...