Enolization rates control mono- versus di-fluorination of 1,3-dicarbonyl derivatives†
Abstract
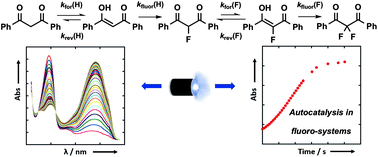
Fluorine-containing 1,3-dicarbonyl derivatives are essential building blocks for drug discovery and manufacture. To understand the factors that determine selectivity between mono- and di-fluorination of 1,3-dicarbonyl systems, we have performed kinetic studies of keto–enol tautomerism and fluorination processes. Photoketonization of 1,3-diaryl-1,3-dicarbonyl derivatives and their 2-fluoro analogues is coupled with relaxation kinetics to determine enolization rates. Reaction additives such as water accelerate enolization processes, especially of 2-fluoro-1,3-dicarbonyl systems. Kinetic studies of enol fluorination with Selectfluor™ and NFSI reveal the quantitative effects of 2-fluorination upon enol nucleophilicity towards reagents of markedly different electrophilicity. Our findings have important implications for the synthesis of α,α-difluoroketonic compounds, providing valuable quantitative information to aid in the design of fluorination and difluorination reactions.


 Please wait while we load your content...
Please wait while we load your content...