Fabrication of rigidity and space variable protein oligomers with two peptide linkers†
Abstract
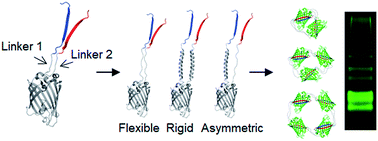
Supramolecular protein assemblies have garnered considerable interest due to their potential in diverse fields with unrivaled attainable functionalities and structural accuracy. Despite significant advances in protein assembly strategies, inserting long linkers with varied lengths and rigidity between assembling protein building blocks remains extremely difficult. Here we report a series of green fluorescent protein (GFP) oligomers, where protein building blocks were linked via two independent peptide strands. Assembling protein units for this two-peptide assembly were designed by flopped fusion of three self-assembling GFP fragments with two peptide linkers. Diverse flexible and rigid peptide linkers were successfully inserted into high-valent GFP oligomers. In addition, oligomers with one flexible linker and one rigid linker could also be fabricated, allowing more versatile linker rigidity control. Linker length could be varied from 10 amino acids (aa) even up to 76 aa, which is the longest among reported protein assembling peptide linkers. Discrete GFP oligomers containing diverse linkers with valencies between monomers to decamers were monodispersely purified by gel elution. Furthermore, various functional proteins could be multivalently fused to the present GFP oligomers. Binding assays, size exclusion chromatography, dynamic light scattering, circular dichroism, differential scanning calorimetry, and transmission electron microscopy suggested circular geometries of the GFP oligomers and showed distinct characteristics of GFP oligomers with length/rigidity varied linkers. Lastly, a surface binding study indicated that more spaced oligomeric binding modules offered more effective multivalent interactions than less spaced modules.

 Please wait while we load your content...
Please wait while we load your content...