Enantioselective construction of the tricyclic core of curcusones A–D via a cross-electrophile coupling approach†
Abstract
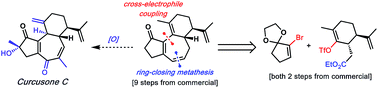
Herein we report our recent progress toward the enantioselective total synthesis of the diterpenoid natural products curcusones A–D by means of complementary Stetter annulation or ring-closing metathesis (RCM) disconnections. Using the latter approach, we have achieved the concise construction of the 5–7–6 carbocyclic core embedded in each member of the curcusone family. Essential to this route is the use of a cross-electrophile coupling strategy, which has not previously been harnessed in the context of natural product synthesis.


 Please wait while we load your content...
Please wait while we load your content...