Dimeric boroles: effective sources of monomeric boroles for heterocycle synthesis†
Abstract
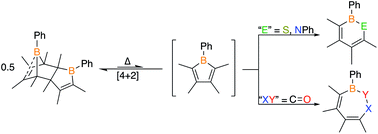
Monomeric boroles have been gaining attention as reagents for the synthesis of heterocycles due to their ability to insert atoms into the BC4 ring in a single step. Although unique boron frameworks can be accessed via this methodology, the products feature aryl substitution on the carbon centers as steric bulk is required to preclude borole dimerization. This work demonstrates that insertion chemistry is possible with Diels–Alder dimeric boroles and that such reactivity is not exclusive to monomeric boroles with bulky groups. With 1-phenyl-2,3,4,5-tetramethylborole dimer, the formal 1,1-insertion of a nitrene and sulfur generate the six-membered aromatic 1,2-azaborine and 1,2-thiaborine, respectively. The isolation of the 1,2-thiaborine enabled the synthesis of an η6-chromium complex. Benzophenone and diphenylketene readily insert a CO unit to generate BOC5 seven-membered rings confirming dimeric boroles can serve as monomeric synthons in 1,2-insertion reactions. An epoxide did not furnish the anticipated eight-membered BOC6 ring, instead provided a bicyclic system with a BOC3 ring. The insertion chemistry was demonstrated with two other borole dimers featuring different substitution with diphenylketene as a substrate. This work elevates borole insertion chemistry to a new level to access products that do not require bulky substitution.


 Please wait while we load your content...
Please wait while we load your content...