Ketoxime peptide ligations: oxidative couplings of alkoxyamines to N-aryl peptides†
Abstract
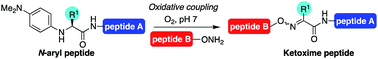
Chemoselective ligation methods that preserve or introduce side chain diversity are critical for chemical synthesis of peptides and proteins. Starting from ketone substrates instead of aldehydes in oxime ligation reactions would allow substitution at the site of ligation; however, synthetic challenges to readily access ketone derivatives from common amino acid building blocks have precluded the widespread use of ketoxime peptide ligation reactions thus far. Moreover, ketones are typically much slower to react in condensation reactions compared to aldehydes. Here, one-pot catalyst-free oxidative couplings of α-substituted N-aryl peptides with alkoxyamines provide access to oxime linkages with diverse side chains. Electron-rich N-(p-Me2N-phenyl)-amino acids possessing substituents at the α-carbon were found to be uniquely capable of undergoing site-selective α-C–H oxidations in situ under an O2 atmosphere at neutral pH. Comparative studies with N-arylglycinyl peptides revealed that substitution at the α-carbon caused notable changes in reactivity, with greater sensitivity to solvent and buffer salt composition.


 Please wait while we load your content...
Please wait while we load your content...