A general synthesis of dendralenes†
Abstract
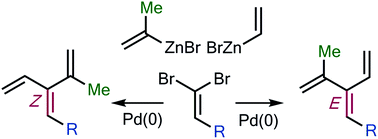
The first general synthetic approach to substituted [3]- and higher dendralenes is reported. Fifty-one mono- through to penta-substituted dendralenes carrying alkyl-, cycloalkyl-, alkenyl-, alkynyl-, aryl- and heteroaryl-substitutents are accessed, and the first (E)/(Z)-stereoselective syntheses of dendralenes are reported (twenty-eight examples). The approach involves twofold Pd(0)-catalyzed Negishi couplings of 1,1-dibromoalkenes with alkenylzinc reagents, and exploits both substrate- and catalyst-controlled aspects of chemo-, regio- and stereoselectivity in the two C(sp2)–C(sp2) bond forming steps. The value of the new hydrocarbons in rapid structural complexity generation is demonstrated through their deployment in unprecedented diene- and triene-transmissive pericyclic reaction sequences.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...