A mild catalytic synthesis of 2-oxazolines via oxetane ring-opening: rapid access to a diverse family of natural products†
Abstract
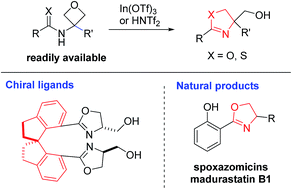
A new catalytic protocol for the expedient synthesis of oxazolines from oxetanes is disclosed. This mild process complements the conventional oxazoline synthesis based on non-catalytic cyclization of β-hydroxy or unsaturated amides. It is also a new addition to the reactivity profile of oxetanes leading to heterocycles. In the presence of In(OTf)3, various 3-amido oxetanes underwent smooth intramolecular cyclization to form the corresponding 2-oxazolines, including some valuable oxazoline-based bidentate ligands. This protocol also provides rapid access to various natural products and antibacterial molecules.


 Please wait while we load your content...
Please wait while we load your content...