Cysteine-to-lysine transfer antibody fragment conjugation†
Abstract
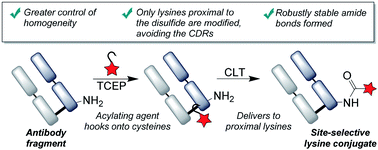
The modification of lysine residues with acylating agents has represented a ubiquitous approach to the construction of antibody conjugates, with the resulting amide bonds being robustly stable and clinically validated. However, the conjugates are highly heterogeneous, due to the presence of numerous lysines on the surface of the protein, and greater control of the sites of conjugation are keenly sought. Here we present a novel approach to achieve the targeted modification of lysines distal to an antibody fragment's binding site, using a disulfide bond as a temporary ‘hook’ to deliver the acylating agent. This cysteine-to-lysine transfer (CLT) methodology offers greatly improved homogeneity of lysine conjugates, whilst retaining the advantages offered by the formation of amide linkages.


 Please wait while we load your content...
Please wait while we load your content...