Palladium(0)/benzoic acid catalysis merges sequences with D2O-promoted labelling of C–H bonds†
Abstract
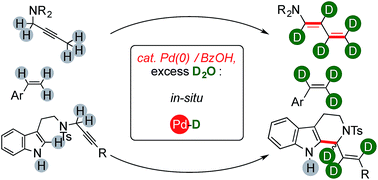
The combination of a Pd(0) complex with benzoic acid in the presence of D2O enables the synthesis of valuable families of highly deuterated organics through elaborate sequential reactions. The catalytic system can convert 2-butyne fragments into the corresponding d-dienamides, which can then readily deliver labeled polycyclic quinone motifs. Propargylated tryptamines lead to formation of highly enriched tetrahydrocarbolines through the C–H activation of their unprotected indole ring. Mechanistic studies reveal the ordered series of events that regulate the outcome of these complex reactions, which include multiple, sequential and selective H/D scrambling from the cheapest and safest deuterium source.


 Please wait while we load your content...
Please wait while we load your content...