Rational design of a multifunctional molecular dye for dual-modal NIR-II/photoacoustic imaging and photothermal therapy†
Abstract
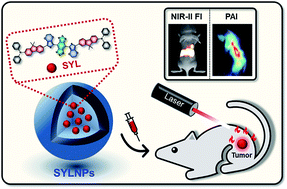
Small-molecule based multifunctional probes play significant roles in biomedical science and possess high clinical translational ability. However, the preparation of these promising probes without complicated synthetic procedures remains a challenging task. Herein, we rationally designed a high-performance DD–A–DD scaffold molecular dye (SYL) with an intrinsic multifunctional ability and then incorporated it into DSPE-mPEG5000 to facilely construct biocompatible NIR-II fluorescent and photoacoustic (PA) dual-modal theranostic nanoprobes (SYL NPs) (∼120 nm). In vivo studies confirmed that SYL NPs exhibited bright NIR-II fluorescence and PA signals in the tumor region with a promising signal to background ratio (S/B). Meanwhile, SYL NPs demonstrated significantly inhibited tumor growth under laser irradiation with no noticeable side effects. These promising results highlighted SYL NPs as a potential theranostic platform for cancer diagnosis (NIR-II region) and therapy.
- This article is part of the themed collections: Most popular 2019-2020 analytical chemistry articles, Celebrating Chemical Science in Korea, The Mechanics of Supramolecular Chemistry and Most popular 2018-2019 analytical chemistry articles


 Please wait while we load your content...
Please wait while we load your content...