Pd-catalyzed site-selective C(sp2)–H radical acylation of phenylalanine containing peptides with aldehydes†
Abstract
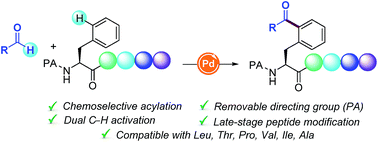
The site-selective functionalization of C–H bonds within a peptide framework remains a challenging task of prime synthetic importance. Herein, the first Pd-catalyzed δ-C(sp2)–H acylation of Phe containing peptides with aldehydes is described. This oxidative coupling is distinguished by its site-specificity, tolerance of sensitive functional groups, scalability, and enantiospecificity and exhibits entire chemoselectivity for Phe motifs over other amino acid units. The compatibility of this dehydrogenative acylation platform with a number of oligopeptides of high structural complexity illustrates its ample opportunities for the late-stage peptide modification and bioconjugation.
- This article is part of the themed collection: Editor's choice - Paolo Melchiorre


 Please wait while we load your content...
Please wait while we load your content...