Enantioselective synthesis of quaternary 3,4-dihydroisoquinolinones via Heck carbonylation reactions: development and application to the synthesis of Minalrestat analogues†
Abstract
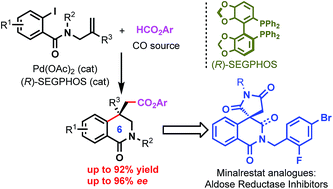
Minalrestat and its analogues represent structurally novel aldose reductase inhibitors, and the asymmetric synthesis of such pharmaceutically privileged molecules has not been reported yet. We have developed a palladium-catalyzed enantioselective intramolecular carbonylative Heck reaction by using formate esters as the source of CO, which represents the first enantioselective synthesis of quaternary 3,4-dihydroisoquinolines. The reaction provides a facile and efficient method for the synthesis of enantiopure nitrogen-containing heterocyclic compounds bearing an all-carbon quaternary stereocenter. The reaction has been successfully applied to the first asymmetric synthesis of Minalrestat analogues.


 Please wait while we load your content...
Please wait while we load your content...