Enantioselective carbene insertion into the N–H bond of benzophenone imine†
Abstract
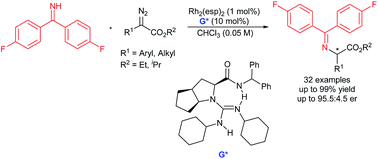
Efficient enantioselective insertion of α-diazoesters into the N–H bond of N-sp2-hybridized benzophenone imine was realized by using Rh2(esp)2 and chiral guanidine cooperative catalysis. Both aliphatic and aromatic substituted α-amino esters were obtained in high yields (up to 99%) and good enantioselectivities (up to 95.5 : 4.5 er) under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...