Reversal of reaction type selectivity by Lewis acid coordination: the ortho photocycloaddition of 1- and 2-naphthaldehyde†
Abstract
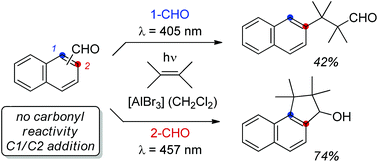
The value of a specific substrate class for synthetic applications is greatly enhanced if different types of reactions can be performed selectively upon a judicious choice of reaction conditions. In the present study it was shown that the typical photochemical behaviour of naphthaldehydes is completely altered in the presence of Lewis acids. Without Lewis acids, reactions at the carbonyl group are exclusively observed while Lewis acids facilitate a visible light-mediated cycloaddition at the arene core providing access to products of aromatic C–H functionalization via cyclobutane intermediates.


 Please wait while we load your content...
Please wait while we load your content...