Selective access to either a doubly boron-doped tetrabenzopentacene or an oxadiborepin from the same precursor†
Abstract
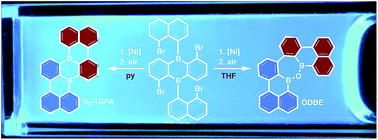
The well-known red emitter tetrabenzo[de,hi,op,st]pentacene (TBPA) has been transformed into a bright blue emitter (B2-TBPA; λem = 472 nm; c-hexane) via substitutional doping with two boron atoms. In contrast to the electron-rich TBPA, which forms endo-peroxides with O2 under daylight, the benchtop-stable B2-TBPA is a good electron acceptor and undergoes reversible reduction at a moderate half-wave potential of E1/2 = −1.73 V (vs. FcH/FcH+; THF). Although the size of B2-TBPA falls within the nanoscale, the helically twisted compound readily dissolves in c-hexane and does not require solubilizing substituents. The synthesis of B2-TBPA is based on the nickel-mediated Yamamoto-type dehalogenation of tetrabrominated 9,10-di(naphth-1-yl)-9,10-dihydro-9,10-diboraanthracene. This intramolecular C–C heterocoupling reaction shows a remarkable solvent dependence: B2-TBPA forms only in pyridine (79% yield), whereas an oxadiborepin is obtained from THF solutions (ODBE, 81%; the reaction mixture is quenched with air in both cases). Insight into the corresponding reaction mechanism was gained from the isolation of intermediates and an investigation of their chemical properties. ODBE is an interesting blue emitter in its own right. Furthermore, it can be ring-opened with excess BBr3 at the B–O–B moiety to afford a dimeric borabenzo[de]anthracene.


 Please wait while we load your content...
Please wait while we load your content...