Photoredox-catalyzed stereoselective alkylation of enamides with N-hydroxyphthalimide esters via decarboxylative cross-coupling reactions†
Abstract
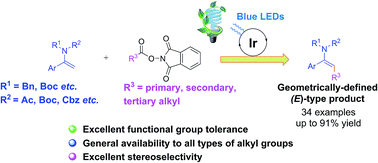
Stereoselective β-C(sp2)–H alkylation of enamides with redox-active N-hydroxyphthalimide esters via a photoredox-catalyzed decarboxylative cross-coupling reaction is demonstrated. This methodology features operational simplicity, broad substrate scopes, and excellent stereoselectivities and functional group tolerance, affording a diverse array of geometrically defined and synthetically valuable enamides bearing primary, secondary or tertiary alkyl groups in satisfactory yields.


 Please wait while we load your content...
Please wait while we load your content...