Metal-free C–C bond formation via coupling of nitrile imines and boronic acids†
Abstract
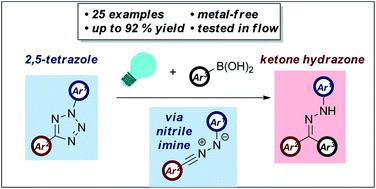
The challenges of developing sustainable methods of carbon–carbon bond formation remains a topic of considerable importance in synthetic chemistry. Capitalizing on the highly reactive nature of the nitrile imine 1,3-dipole, we have developed a novel metal-free coupling of this species with aryl boronic acids. Photochemical generation of a nitrile imine intermediate and trapping with a palette of boronic acids enabled rapid and facile access to a broad library of more than 25 hydrazone derivatives in up to 92% yield, forming a carbon–carbon bond in a metal free fashion. This represents the first reported example of direct reaction between boronic acids and a 1,3-dipole.


 Please wait while we load your content...
Please wait while we load your content...