Oxygen atom transfer with organofunctionalized polyoxovanadium clusters: O-atom vacancy formation with tertiary phosphanes and deoxygenation of styrene oxide†
Abstract
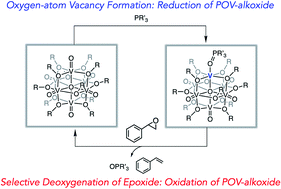
We report a rare example of oxygen atom transfer (OAT) from a polyoxometalate cluster to a series of tertiary phosphanes. Addition of PR3 (PR3 = PMe3, PMe2Ph, PMePh2, PPh3) to a neutral methoxide-bridged polyoxovanadate-alkoxide (POV-alkoxide) cluster, [V6O7(OMe)12]0, results in isolation of a reduced structure with phosphine oxide datively coordinated to a site-differentiated VIII ion. A positive correlation between the steric and electronic properties of the phosphane and the reaction rate was observed. Further investigation of the steric influence of the alkoxy-bridged clusters on OAT was probed through the use of POV clusters with bridging alkoxide ligands of varying chain length ([V6O7(OR′)12]; R′ = Et, nPr). These investigations expose that steric hinderance of the vanadyl moieties has significant influence on the rate of OAT. Finally, we report the reactivity of the reduced POV-alkoxide clusters with styrene oxide, resulting in the deoxygenation of the substrate to generate styrene. This result is the first example of epoxide deoxygenation using homometallic polyoxometalate clusters, demonstrating the potential for mono-vacant Lindqvist clusters to catalyze the removal of oxygen atoms from organic substrates.


 Please wait while we load your content...
Please wait while we load your content...