Installation of internal electric fields by non-redox active cations in transition metal complexes†
Abstract
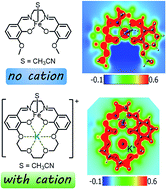
Local electric fields contribute to the high selectivity and catalytic activity in enzyme active sites and confined reaction centers in zeolites by modifying the relative energy of transition states, intermediates and/or products. Proximal charged functionalities can generate equivalent internal electric fields in molecular systems but the magnitude of their effect and impact on electronic structure has been minimally explored. To generate quantitative insight into installing internal fields in synthetic systems, we report an experimental and computational study using transition metal (M1) Schiff base complexes functionalized with a crown ether unit containing a mono- or dicationic alkali or alkaline earth metal ion (M2). The synthesis and characterization of the complexes M1 = Ni(II) and M2 = Na+ or Ba2+ are reported. The electronic absorption spectra and density functional theory (DFT) calculations establish that the cations generate a robust electric field at the metal, which stabilizes the Ni-based molecular orbitals without significantly changing their relative energies. The stabilization is also reflected in the experimental Ni(II/I) reduction potentials, which are shifted 0.12 V and 0.34 V positive for M2 = Na+ and Ba2+, respectively, compared to a complex lacking a proximal cation. To compare with the cationic Ni complexes, we also synthesized a series of Ni(salen) complexes modified in the 5′ position with electron-donating and -withdrawing functionalities (–CF3, –Cl, –H, –tBu, and –OCH3). Data from this series of compounds provides further evidence that the reduction potential shifts observed in the cationic complexes are not due to inductive ligand effects. DFT studies were also performed on the previously reported monocationic and dicatonic Fe(II)(CH3CN) and Fe(III)Cl analogues of this system to analyze the impact of an anionic chloride on the electrostatic potential and electronic structure of the Fe site.


 Please wait while we load your content...
Please wait while we load your content...