Accurate quantum chemical energies for 133 000 organic molecules†
Abstract
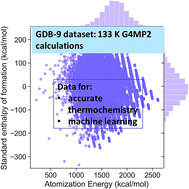
The energies of the 133 000 molecules in the GDB-9 database have been calculated at the G4MP2 level of theory and then were used to calculate their enthalpies of formation. This database contains organic molecules having nine or less atoms of carbon, nitrogen, oxygen, and fluorine, as well as hydrogen atoms. The accuracy of the G4MP2 energies was investigated on a subset of 459 of the molecules having experimental enthalpies of formation with small uncertainties. On this subset the G4MP2 enthalpies of formation have an accuracy of 0.79 kcal mol−1, which is similar to its accuracy previously reported for the smaller G3/05 test set. An error analysis of the theoretical enthalpies of formation of the 459 molecules is presented in terms of the size and type of the molecules. Three different density functionals (B3LYP, ωB97X-D, M06-2X) were also assessed on 459 molecules of accurate enthalpy data for comparison with the G4MP2 results. The G4MP2 energies for the 133 K molecules provide a database that can be used to calculate accurate reaction energies as well as to assess new or existing experimental enthalpies of formation. Several examples are given of types of reactions that can be predicted using the G4MP2 database of energies. The G4MP2 energies of the GDB-9 molecules will also be useful in future investigations of applications of machine learning to quantum chemical data.


 Please wait while we load your content...
Please wait while we load your content...