Highly stereoselective nickel-catalyzed difluoroalkylation of aryl ketones to tetrasubstituted monofluoroalkenes and quaternary alkyl difluorides†
Abstract
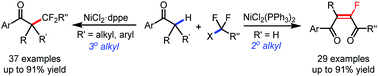
A nickel-catalyzed difluoroalkylation of α-C–H bonds of aryl ketones to furnish highly stereo-defined tetrasubstituted monofluoroalkenes or quaternary alkyl difluorides from secondary or tertiary ketones, respectively, has been established. Mechanistic investigations indicated that these C–H fluoroalkylations proceed via a Ni(I)/Ni(III) catalytic cycle. An obvious fluorine effect was observed in the reaction, and this reaction has demonstrated high stereoselectivity, mild conditions, and broad substrate scopes, thus enabling the late-stage fluoroalkylation of bioactive molecules. This method offers a solution for expedient construction of monofluoroalkenes from readily available materials, and provides an efficient approach for the synthesis of bioactive fluorinated compounds for the discovery of lead compounds in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...