Redox-neutral ortho-C–H amination of pinacol arylborates via palladium(ii)/norbornene catalysis for aniline synthesis†
Abstract
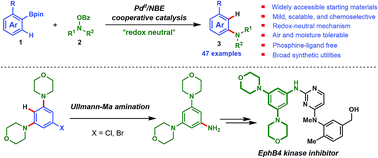
A palladium(II)/norbornene cooperative catalysis enabled redox-neutral ortho-C–H amination of pinacol aryl- or heteroarylborates for the synthesis of structurally diverse anilines is reported. This method is scalable, robust (tolerance of air and moisture), phosphine ligand-free, and compatible with a wide range of functionalities. These practical features make this reaction amenable for industry. A plethora of synthetically very useful halogenated anilines, which often cannot be prepared via other transition-metal-catalyzed aminations, are readily produced using this method. Particularly, the orthogonal reactivity between pinacol arylborates and aryl iodides is demonstrated. Preliminary deuterium-labeling studies reveal a redox-neutral ipso-protonation mechanism of this process, which will surely inspire the future development of this field. Overall, the exceptionally broad scope (47 examples) and reliability of this procedure, together with the wide availability of pinacol arylborates, make this chemistry a valuable addition to the existing methods for aniline synthesis.


 Please wait while we load your content...
Please wait while we load your content...