A sterically encumbered photoredox catalyst enables the unified synthesis of the classical lignan family of natural products†
Abstract
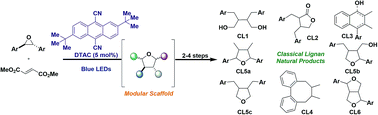
Herein, we detail a unified synthetic approach to the classical lignan family of natural products that hinges on divergence from a common intermediate that was strategically identified from nature's biosynthetic blueprints. Efforts toward accessing the common intermediate through a convergent and modular approach resulted in the discovery of a sterically encumbered photoredox catalyst that can selectively generate carbonyl ylides from electron-rich epoxides. These can undergo concerted [3 + 2] dipolar cycloadditions to afford tetrahydrofurans, which were advanced (2–4 steps) to at least one representative natural product or natural product scaffold within all six subtypes in classical lignans. The application of those synthetic blueprints to the synthesis of heterolignans bearing unnatural functionality was demonstrated, which establishes the potential of this strategy to accelerate structure–activity-relationship studies of these natural product frameworks and their rich biological activity.


 Please wait while we load your content...
Please wait while we load your content...