Synthesis of montbretin A analogues yields potent competitive inhibitors of human pancreatic α-amylase†
Abstract
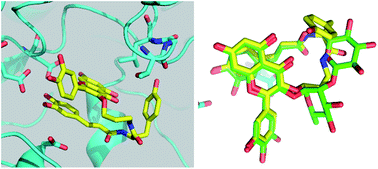
Simplified analogues of the potent human amylase inhibitor montbretin A were synthesised and shown to bind tightly, KI = 60 and 70 nM, with improved specificity over medically relevant glycosidases, making them promising candidates for controlling blood glucose. Crystallographic analysis confirmed similar binding modes and identified new active site interactions.


 Please wait while we load your content...
Please wait while we load your content...