Targeting trimeric transmembrane domain 5 of oncogenic latent membrane protein 1 using a computationally designed peptide†
Abstract
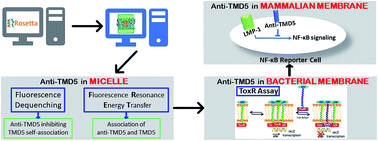
Protein–protein interactions are involved in diverse biological processes. These interactions are therefore vital targets for drug development. However, the design of peptide modulators targeting membrane-based protein–protein interactions is a challenging goal owing to the lack of experimentally-determined structures and efficient protocols to probe their functions. Here we employed rational peptide design and molecular dynamics simulations to design a membrane-insertable peptide that disrupts the strong trimeric self-association of the fifth transmembrane domain (TMD5) of the oncogenic Epstein–Barr virus (EBV) latent membrane protein-1 (LMP-1). The designed anti-TMD5 peptide formed 1 : 2 heterotrimers with TMD5 in micelles and inhibited TMD5 oligomerization in bacterial membranes. Moreover, the designed peptide inhibited LMP-1 homotrimerization based on NF-κB activity in EVB positive lymphoma cells. The results indicated that the designed anti-TMD5 peptide may represent a promising starting point for elaboration of anti-EBV therapeutics via inhibition of LMP-1 oligomerization. To the best of our knowledge, this represents the first example of disrupting homotrimeric transmembrane helices using a designed peptide inhibitor.


 Please wait while we load your content...
Please wait while we load your content...