Active template rotaxane synthesis through the Ni-catalyzed cross-coupling of alkylzinc reagents with redox-active esters†
Abstract
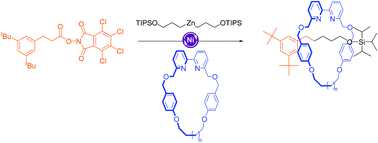
The synthesis of unsymmetrical axle [2]rotaxanes through a recently developed Ni-catalyzed C(sp3)–C(sp3) cross-coupling of redox-active esters (formed directly from carboxylic acids) and organozinc reagents (derived from alkyl bromides) is reported. The method also furnishes, as a minor product, the symmetrical axle [2]rotaxanes resulting from the homo-coupling of the organozinc half-thread. The rotaxanes are formed in up to 56% yield with the ratio of unsymmetrical rotaxane increasing with the cavity size of the macrocycle. In the absence of the redox-active ester neither rotaxane is formed, even though the homo-coupling rotaxane product does not incorporate the redox-active ester building block. A Ni(III) intermediate is consistent with these observations, providing support for the previously postulated mechanism of the Ni-catalyzed cross-coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...