Far-red/near-infrared emitting, two-photon absorbing, and bio-stable amino-Si-pyronin dyes†
Abstract
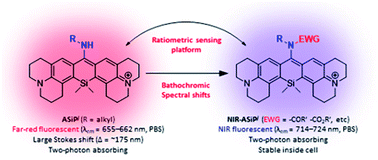
Organic fluorophores emitting in the far-red/near-infrared (NIR) wavelength region are in great demand for minimal autofluorescence and reduced light scattering in deep tissue or whole body imaging. Currently, only a few classes of far-red/NIR fluorophores are available including widely used cyanine dyes, which are susceptible to photobleaching and form nonfluorescent aggregates. Even rare are those far-red/NIR emitting dyes that have two-photon imaging capability. Here we report a new class of far-red/NIR-emitting dyes that are photo-stable, very bright, biocompatible, and also two-photon absorbing. The introduction of an electron-withdrawing group such as N-acyl or N-alkoxycarbonyl groups on the C-10-amino substituent of the new julolidine-derived amino-Si-pyronin dyes (ASiPj), which emit in the far-red region, causes large bathochromic shifts, leading to NIR-emitting amino-Si-pyronin dyes (NIR-ASiPj) having high cellular stability. Furthermore, the ASiPj–NIR-ASiPj couple offers a novel ratiometric bioimaging platform with a large spectral gap, as demonstrated here with a boronate-containing NIR-ASiPj derivative that is converted to the corresponding ASiPj dye upon reaction with hydrogen peroxide.
- This article is part of the themed collections: Near-infrared (NIR) luminescent probes for bioimaging and biosensing and Celebrating Chemical Science in Korea


 Please wait while we load your content...
Please wait while we load your content...