Using chirality to influence supramolecular gelation†
Abstract
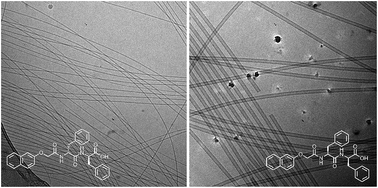
Most low molecular weight gelators are chiral, with racemic mixtures often unable to form gels. Here, we show an example where all enantiomers, diastereomers and racemates of a single functionalized dipeptide can form gels. At high pH, different self-assembled aggregates are formed and these directly template the structures formed in the gel. Hence, solutions and gels with different properties can be accessed simply by varying the chirality. This opens up new design rules for the field.
- This article is part of the themed collection: Editor’s Choice – Subi George


 Please wait while we load your content...
Please wait while we load your content...