A molecular design strategy toward enzyme-activated probes with near-infrared I and II fluorescence for targeted cancer imaging†
Abstract
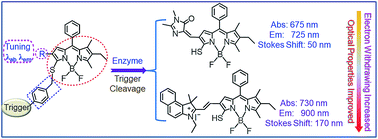
The advance of cancer imaging requires innovations to establish novel fluorescent scaffolds that are excitable and emit in the near-infrared region with favorable Stokes shifts. Nevertheless, the lack of probes with these optimized optical properties presents a major bottleneck in targeted cancer imaging. By coupling of boron dipyrromethene platforms to enzymic substrates via a self-immolative benzyl thioether linker, we here report a strategy toward enzyme-activated fluorescent probes to satisfy these requirements. This strategy is applicable to generate various BODIPY-based probes across the NIR spectrum via introducing diverse electron-withdrawing substituents at the 3-position of the BODIPY core through a vinylene unit. As expected, such designed probes show advantages of two-channel ratiometric fluorescence and light-up NIR (I and II) emission with large Stokes shifts upon enzyme activation, enabling targeted cancer cell imaging and accurate tumor location by real-time monitoring of enzyme activities. This strategy is promising in engineering activatable molecular probes suitable for precision medicine.
- This article is part of the themed collections: Near-infrared (NIR) luminescent probes for bioimaging and biosensing, Most popular 2019-2020 analytical chemistry articles, Most popular 2018-2019 analytical chemistry articles and 2019 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...