Magnesium hydride alkene insertion and catalytic hydrosilylation†
Abstract
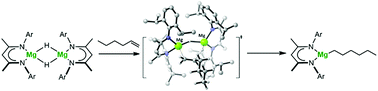
The dimeric β-diketiminato magnesium hydride, [(BDI)MgH]2, reacts at 80 °C with the terminal alkenes, 1-hexene, 1-octene, 3-phenyl-1-propene and 3,3-dimethyl-butene to provide the respective n-hexyl, n-octyl, 3-phenylpropyl and 3,3-dimethyl-butyl magnesium organometallics. The facility for and the regiodiscrimination of these reactions are profoundly affected by the steric demands of the alkene reagent. Reactions with the phenyl-substituted alkenes, styrene and 1,1-diphenylethene, require a more elevated temperature of 100 °C with styrene providing a mixture of the 2-phenylethyl and 1-phenylethyl products over 7 days. Although the reaction with 1,1-diphenylethene yields the magnesium 1,1-diphenylethyl derivative as the sole reaction product, only 64% conversion was achieved over a 21 day timeframe. Reactions with the α,ω-dienes, 1,5-hexadiene and 1,7-octadiene, provided divergent results. The initial 5-alkenyl magnesium reaction product of the shorter chain diene undergoes 5-exo-trig cyclisation via intramolecular carbomagnesiation to provide a cyclopentylmethyl derivative, which was shown by X-ray diffraction analysis to exist as a three-coordinate monomer. In contrast, 1,7-octadiene provided a mixture of two compounds, a magnesium oct-7-en-1-yl derivative and a dimagnesium-octane-1,4-diide, as a result of single or two-fold activation of the terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. The magnesium hydride was unreactive towards internal alkenes apart from the strained bicycle, norbornene, allowing the characterisation of the resultant three-coordinate magnesium norbornyl derivative by X-ray diffraction analysis. Computational analysis of the reaction between [(BDI)MgH]2 and 1-hexene using density functional theory (DFT) indicated that the initial Mg–H/C
C double bonds. The magnesium hydride was unreactive towards internal alkenes apart from the strained bicycle, norbornene, allowing the characterisation of the resultant three-coordinate magnesium norbornyl derivative by X-ray diffraction analysis. Computational analysis of the reaction between [(BDI)MgH]2 and 1-hexene using density functional theory (DFT) indicated that the initial Mg–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C insertion process is rate determining and takes place at the intact magnesium hydride dimer. This exothermic reaction (ΔH = −14.1 kcal mol−1) traverses a barrier of 18.9 kcal mol−1 and results in the rupture of the dinuclear structure into magnesium alkyl and hydride species. Although the latter three-coordinate hydride derivative may be prone to redimerisation, it can also provide a further pathway to magnesium alkyl species through its direct reaction with a further equivalent of 1-hexene, which occurs via a lower barrier of 15.1 kcal mol−1. This Mg–H/C
C insertion process is rate determining and takes place at the intact magnesium hydride dimer. This exothermic reaction (ΔH = −14.1 kcal mol−1) traverses a barrier of 18.9 kcal mol−1 and results in the rupture of the dinuclear structure into magnesium alkyl and hydride species. Although the latter three-coordinate hydride derivative may be prone to redimerisation, it can also provide a further pathway to magnesium alkyl species through its direct reaction with a further equivalent of 1-hexene, which occurs via a lower barrier of 15.1 kcal mol−1. This Mg–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C insertion reactivity provides the basis for the catalytic hydrosilylation of terminal alkenes with PhSiH3, which proceeds with a preference for the formation of the anti-Markovnikov organosilane product. Further DFT calculations reveal that the catalytic reaction is predicated on a sequence of Mg–H/C
C insertion reactivity provides the basis for the catalytic hydrosilylation of terminal alkenes with PhSiH3, which proceeds with a preference for the formation of the anti-Markovnikov organosilane product. Further DFT calculations reveal that the catalytic reaction is predicated on a sequence of Mg–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C insertion and classical Si–H/Mg–C σ-bond metathesis reactions, the latter of which, with a barrier height of 24.9 kcal mol−1, is found to be rate determining.
C insertion and classical Si–H/Mg–C σ-bond metathesis reactions, the latter of which, with a barrier height of 24.9 kcal mol−1, is found to be rate determining.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...